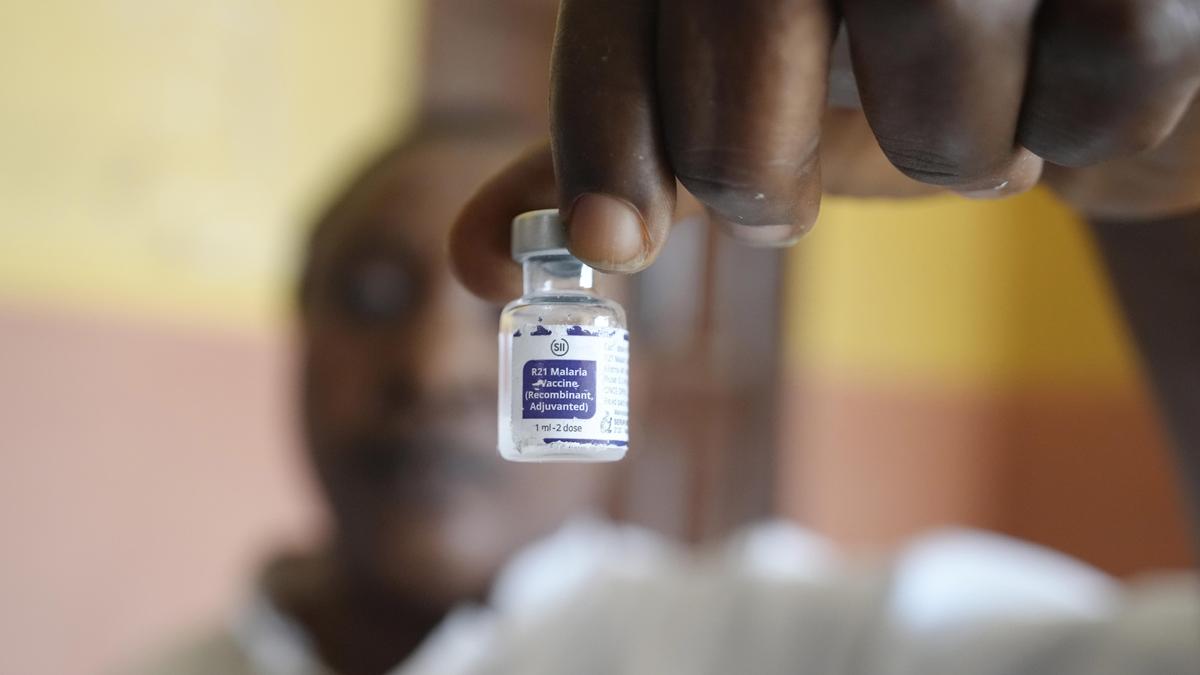
A health worker shows a bottle of the malaria vaccine R21/Matrix-M before administering it to a child at the comprehensive Health Centre in Agudama-Epie, in Yenagoa, Nigeria, Monday, Dec. 9, 2024.
| Photo Credit: AP
Gavi, the Vaccine Alliance and UNICEF have announced a new agreement that it said will make the R21/Matrix-M malaria vaccines significantly more accessible and affordable, paving the way to protect more children.
The deal, backed by Gavi and executed by United Nations Children’s Fund (UNICEF), is expected to generate up to $90 million in savings for Gavi and countries, equivalent to more than 30 million additional doses — enabling the full vaccination of nearly seven million more children against malaria over the next five years. The deal is financed by Gavi through an advance payment enabled by the innovative International Finance Facility for Immunisation (IFFIm) mechanism.
The lower price of the vaccine — at $ 2.99 per dose — is anticipated to take effect in approximately one year.
Gavi facilitates and finances the procurement, logistics, market shaping and integration of malaria vaccines into national immunisation programmes. To date, over 40 million doses of malaria vaccines have been delivered through the Gavi malaria vaccination programme and are now part of routine immunisation in 24 African countries that together represent more than 70% of the world’s malaria burden.
UNICEF is the world’s largest buyer of vaccines, delivering nearly three billion doses of vaccine every year, enough to vaccinate almost half of the world’s children. It also leads and maintains engagement with strategic manufacturers to achieve the best possible prices.
WHO’s prequalified vaccines
WHO has prequalified two malaria vaccines to-date: R21/Matrix-M (co-developed by the University of Oxford and Serum Institute of India, leveraging Novavax’s Matrix-M adjuvant technology] and RTS,S/AS01 (developed by GlaxoSmithKline (GSK), PATH and partners).
Both vaccines are prequalified and recommended by WHO to prevent malaria in children and are safe and effective.
In Phase-3 clinical trials, both vaccines reduced malaria cases by more than half during the first year after vaccination, a period when children are at high risk of illness and death. A fourth dose given in the second year of life prolonged protection.
Both vaccines reduce malaria cases by about 75% when given seasonally in areas of highly seasonal transmission — where half of childhood malaria deaths occur.
Published – November 24, 2025 07:42 pm IST