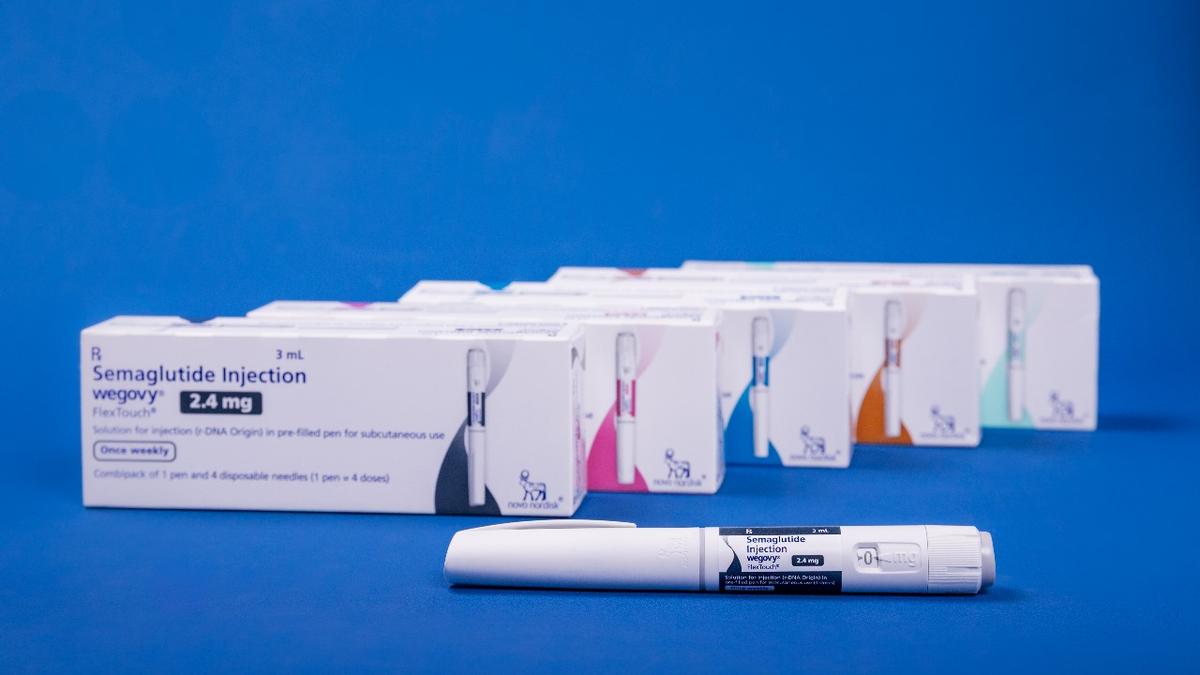
Available in five dosing strengths — 0.25 mg, 0.5 mg, 1 mg, 1.7 mg and a maintenance dose of 2.4 mg — Wegovy is administered through a pen device
| Photo Credit: Special Arrangement
Global pharmaceutical company Novo Nordisk has launched Wegovy (semaglutide 2.4 mg), its once-weekly injectable weight management therapy, in India. Indicated for chronic weight management and reducing the risk of major adverse cardiovascular events (MACE), Wegovy will address both weight loss and cardiovascular outcomes for people living with obesity, representatives from the pharma company said at a press meet on Tuesday, June 24, 2025.
Wegovy comes in five dose levels — 0.25 mg, 0.5 mg, 1 mg, 1.7 mg, and a regular maintenance dose of 2.4 mg — and is delivered using an easy-to-use injection pen. The company has kept the price of the first three dose levels the same to help patients adjust their dosage without extra cost.
In March, American pharmaceutical major Eli Lilly and Company launched its diabetes and obesity management drug Mounjaro (tirzepatide) in India. The company launched the drug in a single-dose vial.
Studies have shown that at least one in three people using Wegovy along with lifestyle changes may lose over 20% of their body weight. It has also been proven to lower the risk of heart attacks, strokes and related deaths by 20% in people who already have heart disease and obesity, the company said.
According to national health data, about 24% of Indian women and 23% of men live with obesity. Experts say this increases the risk of many illnesses and puts a growing burden on the healthcare system.
“Obesity is a long-term health condition that needs medical support,” said Vikrant Shrotriya, managing director, Novo Nordisk India. “
Wegovy is a prescription-only medicine and should be used under medical supervision.
Published – June 24, 2025 05:28 pm IST